
BERLIN-CHEMIE has 18 departments
BERLIN-CHEMIE AG and Menarini GmbH are associate companies based at the same premises. While BERLIN-CHEMIE AG focuses on production and sales, Menarini GmbH predominately focuses on services such as research and administration.
- A. Menarini Diagnostics
With its own field sales force, the A. Menarini Diagnostics department distributes blood-glucose monitors for patient self-testing and large diagnostic appliances for medical laboratories specialising in cancer diagnostics. Its office-based staff handle marketing, customer service and sales management activities.
- Field Sales Force
Our multiple award-winning scientific field sales force advises medical and pharmaceutical professionals in medical practices, clinics and pharmacies on our medicinal products and medical devices. All field sales force staff regularly attend training courses and individual further training programmes to keep their product and specialist knowledge and sales skills up to date.
- Commercial Centre Germany
The Commercial Centre is the commercial heart of our company. It handles order entry, material planning, returns and complaints, as well as invoicing, monitoring and reporting for all national commercial activities. Price databases are also maintained and the switchboard is housed in the Commercial Centre.
- Healthcare Management
Our healthcare managers advise clinic and practice staff on how patient care can be optimised. They analyse health-policy and legal frameworks and organise regional networks in which they exchange information and ideas with health-policy decision-makers, healthcare professionals, associations and healthcare service providers.
- Manufacturing
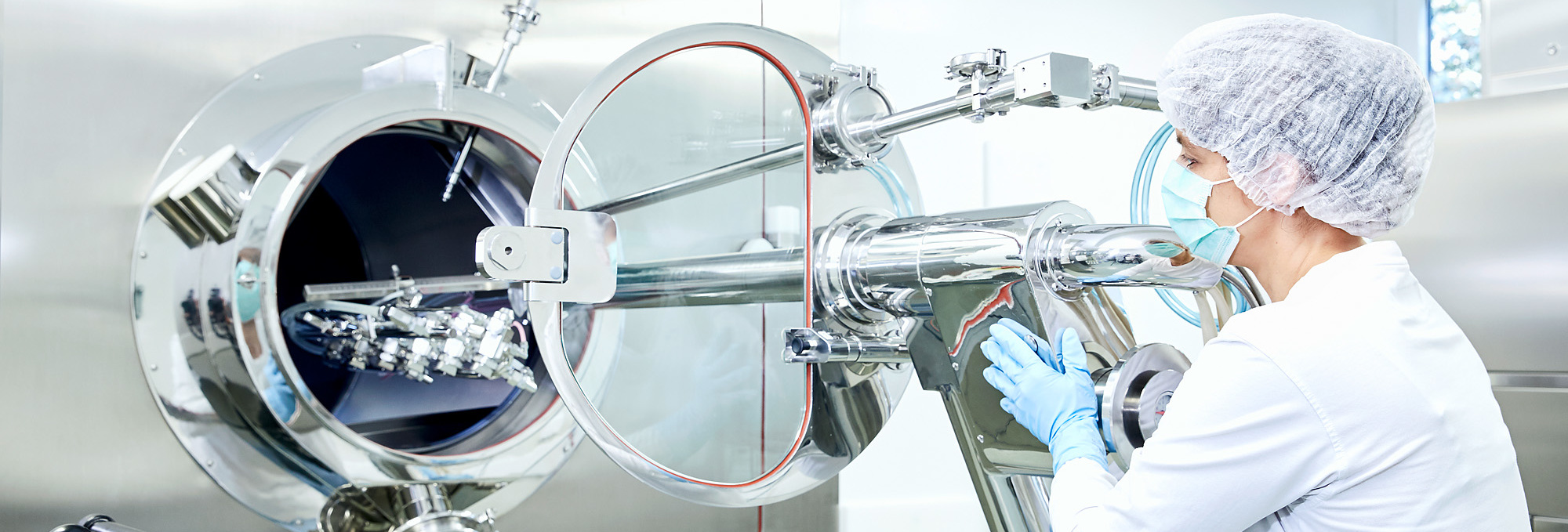
At our Adlershof and Britz productions sites in Berlin, some 350 staff members are responsible for ensuring our products are correctly manufactured and packaged. They operate and monitor automated production equipment and pay strict attention to compliance with the prescribed quality and hygiene standards. They assess, validate and document the manufacturing processes and ensure that products are transferred between the production sites. Solid medicines (tablets, coated tablets, capsules), liquids (solutions and drops), suppositories and infusion solutions are produced.
- International Division
Within the global Menarini Group, BERLIN-CHEMIE AG has subsidiaries or representative offices in more than 30 countries in Central and Eastern Europe and Central Asia. The International Division in Berlin works closely with these country organisations and supports them in areas such as marketing, business development, licensing and exports.
- Key Account Management
In the Key Account Management department, we draft bids, negotiate prices, draw up contracts and manage tenders. We work in partnership with wholesalers, mail-order pharmacies, clinics, health insurance companies and other key accounts.
- Local Drug Safety Unit (LDSU)
To ensure our medicinal products are safe to use, we duly consider the benefits and risks of our products in comprehensive procedures. Authorities, users and internal company stakeholders make informed decisions based on the relevant findings.
- Marketing
With their extensive expertise in health-policy frameworks, our product managers develop suitable concepts for the market launch and marketing of our products, whereby medical information and pharmaceutical marketing go hand in hand.
- Market Research & Business Development
This department focuses on strategies. The market research, business development, pricing and contract management teams collaborate to improve the position of our company on the German pharmaceutical market and make it even more successful, focusing on figures, facts and data, the latest trends and co-operations for new medicinal products.
- Medicine & Research
We continue to oversee medicinal products even once they’re on the market. This involves closely monitoring scientific, social and health-policy developments and trends in order to constantly improve the benefits of the products and keep the relevant patient information leaflets (PILs) up to date. In this department, we also closely examine the causes of and diagnosis and treatment options for diseases.
- Human Resources
In the Human Resources department, we recruit new employees. We also provide staff with fair advice on issues such as employee benefits, labour law, the legal protection of working mothers and parental leave and leave to care for family members. Our colleagues at Menarini GmbH complement the services we provide with services relating to training, payroll and HR marketing activities.
- Quality Assurance
In cooperation with all departments, our Quality Assurance Department checks and optimises the operational processes to ensure the company-wide quality management system, the regulatory documents and the manufactured products meet international standards and are always up to date. Our colleagues therefore collaborate closely with many departments, other group sites, as well as with business partners and authorities in Germany and worldwide.
- Quality Control
Staff in the Quality Control department perform the quality tests dictated by national and international pharmacopoeias and our quality management system. This is how they check, for example, the quality of raw materials, intermediate products, packaging materials and finished medicinal products and develop new analytical methods for drug testing and proving the necessary stability of the medicinal products manufactured.
- Sales Controlling
Efficiency is a top priority for us. To ensure efficient sales, the targeted management of the field sales force is a key success factor. In addition to continuously analysing sales activities, the Sales Controlling department maintains the CRM software and optimally supports our field sales force in its daily work.
- Sales Operations
The Sales Operations department co-ordinates the work between our field sales force and office-based sales staff. It also ensures the availability of work materials and organises training events.
- Training
Our trainers conduct our three-month intensive basic training for our new field sales force staff. In addition to knowledge about our products and medical indications, the course also provides intensive communication and sales training. Our highly experienced trainers additionally ensure that our field sales force staff’s knowledge is always up to date and that they are able to carry out their work competently. They regularly refresh medical expertise and communication skills, as well as product know-how, in the form of regional training sessions – individually and in line with specific requirements.
- Regulatory Affairs
New medicinal products need to be licensed and those already licensed constantly adapted to the latest standards. In close collaboration across the departments, this department compiles the relevant necessary dossiers and submits these to the licensing authorities. What is vital here is scientific and legal expertise. This department also creates and updates product information texts (i.e. Summaries of Product Characteristics [SPCs] and patient information leaflets [PILs], patient information on packaging and labels). These texts need to meet the relevant statutory regulations and be clear and informative for specialists and laymen alike.

Colleagues from more than 070 countries

~028 employees in our
Secure Operations
department

025 million Euro of
investments in environmental
measures since 1992
Menarini GmbH has 13 departments
- Data Protection
The in-house lawyers and IT experts in our data protection department advise Berlin-Chemie and its subsidiaries in Germany and abroad on all legal and technical issues relating to data protection. They work at the interface to pharmaceutical law and also draft and negotiate contractual provisions and declarations of consent relating to data protection (e.g. in international license agreements, IT agreements, Informed Consent Forms in the context of clinical trials).
- Purchasing
The Purchasing department is responsible for purchasing all goods and services relating to our marketing, advertising and conference participation activities. Vehicles for our field sales force and all goods and services not directly associated with the production of medicinal products are also procured by our central Purchasing department. And this for sites in more than 30 countries in Europe and Asia. Goods and services for our production activities are procured by our Materials Management department.
- eMedia
The eMedia administrative department is our cross-departmental competence centre for digital communication and transformation. Our colleagues here advise and support BERLIN-CHEMIE AG and Menarini GmbH in the implementation of digital projects and manage external service providers. The project managers define standards for the quality of digital products and processes and constantly work to improve on these.
- Finance, Accounting & Controlling
In addition to external accounting, with accounts payable, accounts receivable, asset accounting and G/L accounting, this department is also responsible for internal cost and activity accounting, product costing, inventory valuation and investment accounting. The task of Controlling is to ensure our effective operational development and help us shape our corporate strategy. The areas Tax, Treasury and Internal Audit round off the portfolio.
- Research & Development
In collaboration with the Menarini Group’s other research sites, we develop new pharmaceutical products. The Research & Development department in Berlin focuses on the galenic development of medicines, the chemical-analytical development of new active pharmaceutical ingredients and clinic testing.
- Health, Safety, Environment (HSE)
Safety in the workplace and environmental and health protection are a top priority for us. It is imperative that the statutory and internal regulations are strictly adhered to here. For this, the HSE team maintains an extensive integrated management system, regularly informs and trains employees, checks all processes and monitors our technical equipment, enabling it to safeguard and further improve our high standards – for the benefit of our employees and our company.
- Information Communication Technology (ICT)
Without a functioning IT infrastructure, nothing here would run as it should. When it comes to IT, the ICT department provides for a reliable structure, maximum security and optimal applications, thereby ensuring that our business processes are effective. This means that our workflows are always efficient, and users in all departments have competent contact persons for all IT-related issues.
- Logistics
Logistics involves more than transporting goods from one location to another efficiently, professionally and on time. Our professionals in this department also ensure that our raw materials, packaging and products are stored correctly and co-ordinate our co-operation with partners, wholesalers, pharmacies and clinics – in Germany and abroad.
- Organisational Development (OD) & Demand Management (DM)
Our process professionals ensure that our company’s workflows and communication channels function as smoothly as possible. They analyse where there is need for change and help departments optimise their processes. They also look into where the use of the correct technology could further facilitate all of our work.
- Human Resources
In the Human Resources department, we recruit new employees. We also provide staff with fair advice on issues such as employee benefits, labour law, the legal protection of working mothers and parental leave and leave to care for family members. We provide a multitude of services relating to training, payroll and HR marketing activities.
- Legal Affairs
The in-house lawyers in our Legal Affairs department deal with almost all legal issues that regularly crop up in our companies. The legal areas covered range from general contract law and economic, trade and corporate law to general and specific administrative law; pharmaceutical, medical device and social law; competition, patent, trademark, copyright and data protection law; issues relating to construction and environmental law, or transport law with regard to export control; and much more.
- Risk Management
Our Risk Management department is responsible for the timely identification of operational risks and opportunities, analysing these and elaborating solution proposals to minimise risks. This enables us to remain competitive and financially strong. This department is also responsible for ensuring we have adequate professional insurance cover.
- Supply Chain Management
The Supply Chain Management department procures all the raw materials, packaging and intermediate products necessary for the production of medicinal products. To this end, it selects suitable suppliers and systematically strengthens the relationship with them. Our specialists co-ordinate with Sales and Production to optimally define production volumes, to ensure all our country subsidiaries are properly supplied with medicinal products at all times. In addition, the Supply Chain Management department is also responsible for designing and implementing suitable supply chains for new productions.
- Central Technology
Skilled workers, technicians and engineers work in the Central Technology department to keep our pharmaceutical devices, machines, systems and technical equipment maintained. They plan technical investments, secure the supply of energy and water to the company and make sure that the use of all utilities is efficient and environmentally friendly.
JOIN US
Keen and ready to take the first step
Click here for an overview of all the different jobs you can do at BERLIN-CHEMIE AG and Menarini GmbH. What spurs you on?

Hotline
Regardless of what career topics you have a question about: We are here for you and will be happy to help. Just give us a call!
You can reach us between 7:30 am and 5:30 pm from Monday to Thursday and between 7:30 am and 4:00 pm on Friday.


